Ataraxis™ Breast
Next-generation insights for every breast cancer journey.
Ataraxis Breast is a suite of AI-powered clinical tests designed to personalize breast cancer care. Built on multimodal artificial intelligence, the Ataraxis platform integrates digital pathology and clinical data to predict recurrence risk, treatment benefit, and therapy response—supporting precision oncology across the full continuum of early-stage breast cancer.
Complete treatment selection suite for breast cancer patients
Identify patients with low and high risk of breast cancer recurrence in all patient subtypes, even in patients with TNBC and HER2-positive disease
Assess how much adjuvant chemotherapy reduces recurrence risk and find patients who despite high recurrence risk don’t benefit from CT
Identify patients (un)likely to achieve pathologic complete response to neoadjuvant standard of care therapy in all molecular subtypes
Precision oncology reimagined for the next generation
We continue to regularly update our AI models to incorporate real-world data including new therapies and constantly changing standard of care, advancing in ways static assays cannot.
Ataraxis delivers individualized predictions without vague categorization of patients into non-actionable groups. We provide estimates that directly support treatment decisions.
Unlike legacy assays, often limited to HR+/HER2- patients, the Ataraxis Breast portfolio includes tests that support all major breast cancer subtypes.
Ataraxis tests can be ordered as early as immediately after core needle biopsy to provide insights that help plan both adjuvant and neoadjuvant therapy.
Testing does not require additional procedures or special laboratory work. Ataraxis uses standard H&E-stained slides preserving all remaining tissue for downstream testing.
Results are delivered within one business day of slide receipt, compared with weeks-long turnaround for legacy tests. This reduces patient anxiety and enables earlier treatment decisions.
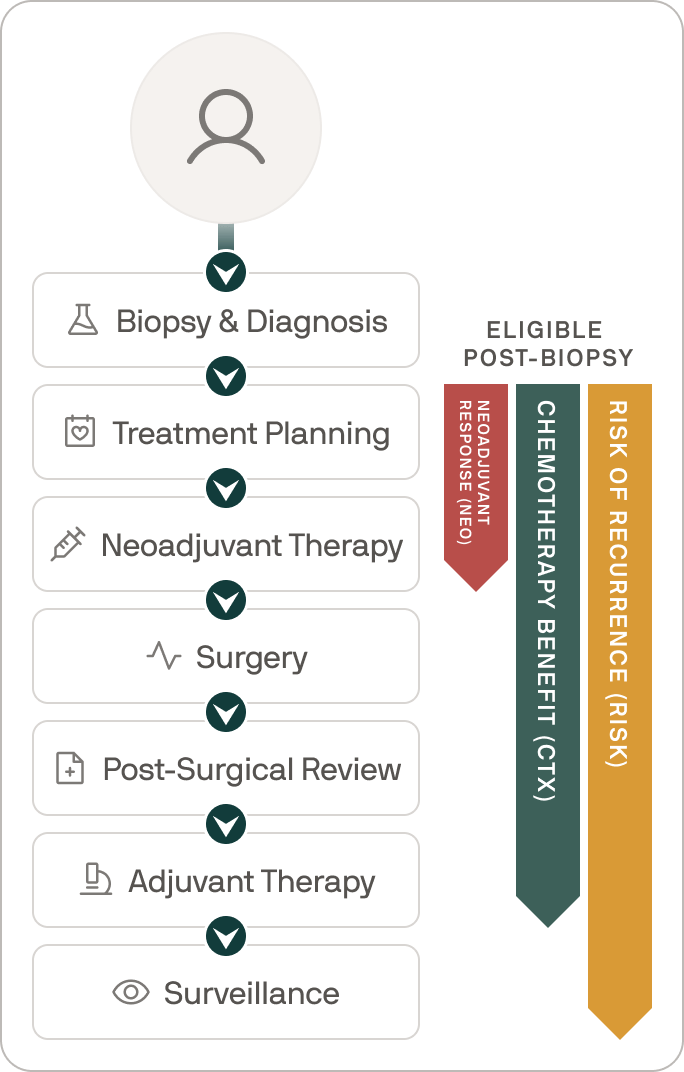
Supporting treatment selection at every step
.png)
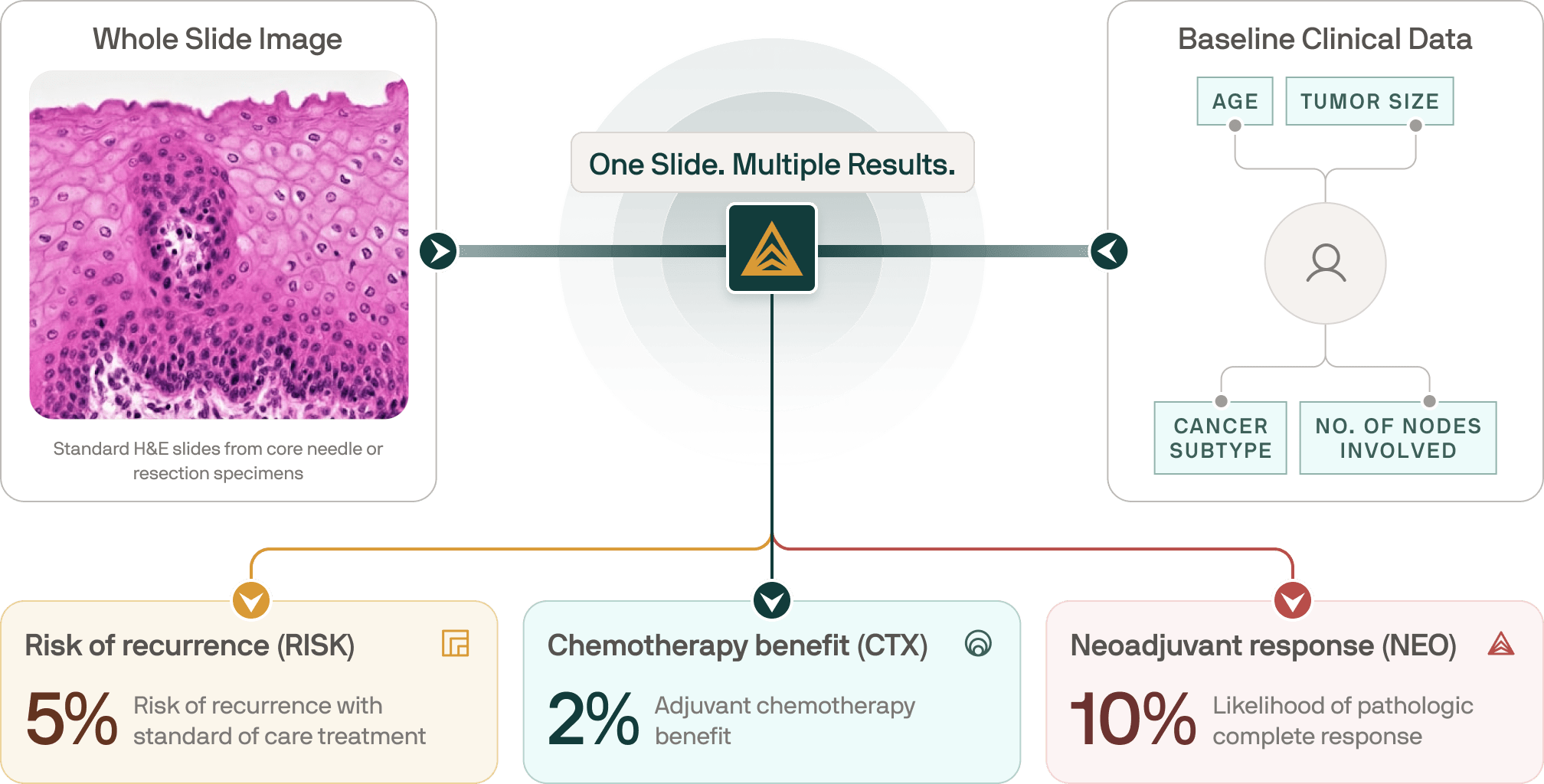
How Ataraxis Breast Works
Ataraxis combines patient-specific and tumor-specific data to generate precise, personalized prognostic and predictive insights. Our models learn thousands of morphology features from routine H&E slides and align them to real-world outcomes, giving clinicians actionable guidance they can trust.
Find out more about Ataraxis Breast
Frequently Asked Questions
Ataraxis Breast tests are intended for women diagnosed with early-stage (I–III) invasive breast cancer. Breast RISK and Breast NEO are available for women with stage I–III invasive breast cancer across all subtypes, including with or without lymph node involvement and regardless of hormone receptor status. Breast CTX is intended for hormone receptor–positive (HR+) breast cancer. Your doctor can confirm which test fits your situation.
These tests provide additional information about risk and prognosis to support conversations with your care team. They don’t choose a treatment for you or replace medical advice. Your doctor will interpret results alongside other clinical information to help tailor next steps.
You can ask once your diagnosis is confirmed—any time before the relevant treatment decision is made—so results can be considered if helpful. In general: NEO is used before decisions about neoadjuvant therapy, CTX before decisions about adjuvant chemotherapy, and RISK can be used as part of treatment planning when results can still inform next steps.
No. These tests are designed to use tissue already collected during your biopsy or surgery.
Your doctor orders the test, and results are sent directly to your doctor to review with you. If you’re a patient and have questions about access, please contact us and we can help guide next steps.
Most results are shared with physicians within one business day once the lab receives the required materials.
Your out-of-pocket cost, if any, depends on your insurance plan. However, Ataraxis is committed to making its innovative tests as affordable and accessible as possible. For this reason, we offer a generous financial assistance program that many patients will qualify for.
Clinicians can order Ataraxis Breast tests through the Ataraxis Ordering Portal. If you don’t yet have an account, contact us and our team will help you get set up.
Make the most informed call for your patients.
Complete treatment selection suite at every stage of your cancer fight
Right after your biopsy, your physician can order Ataraxis Breast and get day-one guidance to help you and care team make confident treatment decisions.
.png)

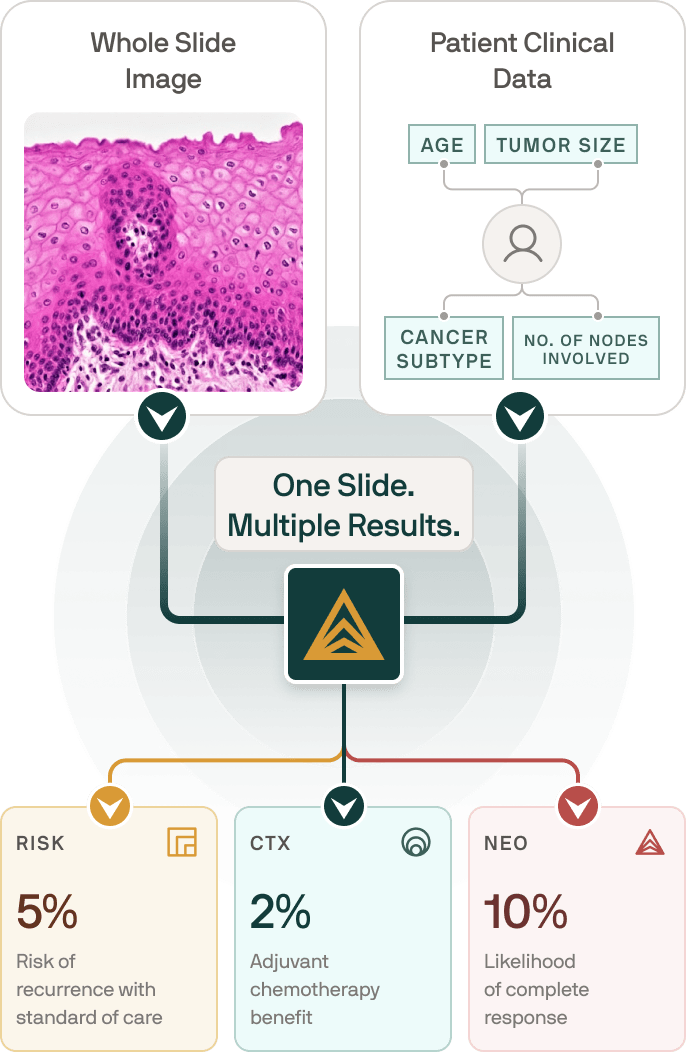
Ataraxis Breast platform is designed to give you and your doctor actionable and more personalized answers at a time when clarity matters most.
How the testing process works
Our platform uses advanced AI technology to analyze your tumor tissue using the same pathology slides collected at the time of your diagnosis. There are no new procedures, no additional appointments, nor extra steps for you.


Here’s exactly what to expect
Ataraxis Breast was developed and validated in patients like you
23,800+
Patients represented in our global development & validation datasets
.png)
Find out more about Ataraxis Breast
Frequently Asked Quesions
Ataraxis Breast tests are intended for women diagnosed with early-stage (I–III) invasive breast cancer. Breast RISK and Breast NEO are available for women with stage I–III invasive breast cancer across all subtypes, including with or without lymph node involvement and regardless of hormone receptor status. Breast CTX is intended for hormone receptor–positive (HR+) breast cancer. Your doctor can confirm which test fits your situation.
These tests provide additional information about risk and prognosis to support conversations with your care team. They don’t choose a treatment for you or replace medical advice. Your doctor will interpret results alongside other clinical information to help tailor next steps.
You can ask once your diagnosis is confirmed—any time before the relevant treatment decision is made—so results can be considered if helpful. In general: NEO is used before decisions about neoadjuvant therapy, CTX before decisions about adjuvant chemotherapy, and RISK can be used as part of treatment planning when results can still inform next steps.
No. These tests are designed to use tissue already collected during your biopsy or surgery.
Your doctor orders the test, and results are sent directly to your doctor to review with you. If you’re a patient and have questions about access, please contact us and we can help guide next steps.
Most results are shared with physicians within one business day once the lab receives the required materials.
Your out-of-pocket cost, if any, depends on your insurance plan. However, Ataraxis is committed to making its innovative tests as affordable and accessible as possible. For this reason, we offer a generous financial assistance program that many patients will qualify for.
Clinicians can order Ataraxis Breast tests through the Ataraxis Ordering Portal. If you don’t yet have an account, contact us and our team will help you get set up.
